2023
Primeros resultados prometedores IN VITRO / IN VIVO
MOLEFY es una spin-off de ARQUIMEA dedicada a buscar una cura para la esclerosis lateral amiotrófica (ELA). La ELA es una enfermedad neurodegenerativa debilitante caracterizada por la pérdida progresiva del control muscular, para la que actualmente no existe un tratamiento eficaz.
A pesar de su baja incidencia, la ELA plantea importantes retos a los sistemas sanitarios debido a la amplia atención médica que se requiere para atender a las personas afectadas. Esto suele traducirse en importantes costes sanitarios, que actualmente superan los 650.000 millones de dólares anuales en gastos médicos y pérdidas económicas.
Aunque la etiología y la fisiopatología exactas de la ELA siguen siendo desconocidas, se ha identificado el TDP-43 como un sello distintivo clave de la enfermedad. Para hacer frente a la urgente necesidad de un tratamiento eficaz, MOLEFY está desarrollando una pequeña molécula denominada AP-2. Este prometedor candidato a fármaco pretende prevenir la muerte de las neuronas motoras invirtiendo los cambios patogénicos del TDP-43.
AP-2 puede atravesar todas las barreras cerebrales y ha demostrado resultados prometedores en recientes ensayos preclínicos con diferentes modelos animales. Estos estudios demostraron que el bloqueo del comportamiento anómalo del TDP-43 no sólo alivia los síntomas de la ELA, sino que también invierte la progresión de la enfermedad.

El planteamiento de MOLEFY está respaldado por datos alentadores obtenidos en modelos celulares tanto animales como de pacientes. AP-2 ha demostrado eficacia y un perfil de seguridad farmacológica favorable en modelos animales con y sin roedores. Nuestro objetivo es reunir toda la información necesaria para iniciar ensayos clínicos en humanos antes de 2025.

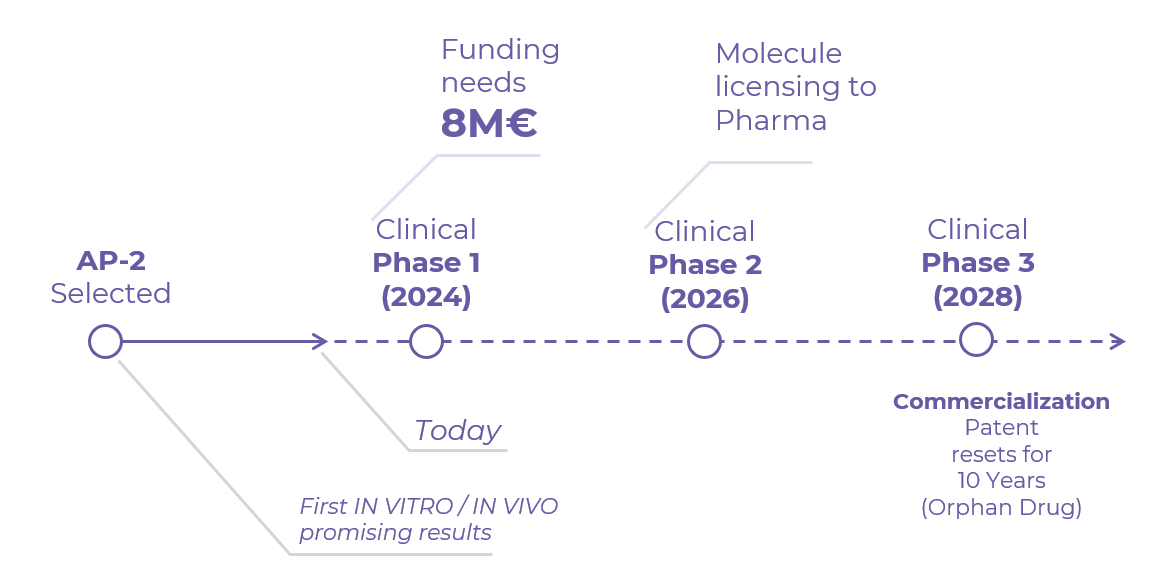
Uno de los principales objetivos de MOLEFY para 2024 es conseguir una ronda de financiación para acelerar el desarrollo de los ensayos clínicos de su molécula más prometedora (AP-2), con el fin de iniciarlos en 2025. Actualmente, se necesitan 1,5 millones de euros para desarrollar la fase I de los ensayos, y se requerirá inversión adicional para las fases II y III.
Hemos reunido un equipo científico y clínico de gran talento comprometidos con el avance de la investigación de la ELA y el desarrollo de tratamientos eficaces. Con una sólida protección de la propiedad intelectual, estamos bien posicionados para ampliar nuestras operaciones y llevar nuestra molécula al mercado.

Primeros resultados prometedores IN VITRO / IN VIVO
Finalización de los estudios preclínicos.
Financiación necesaria: 1.5M$
Estudios regulatorios para la fase preclínica
Lanzamiento del fármaco
La patente se renueva 10 años (medicamento huérfano)
Además, estamos desarrollando un marco basado en IA para generar modelos predictivos que analicen las complejas relaciones entre estructuras moleculares, características bioquímicas y propiedades farmacológicas de nuestro candidato a fármaco.
Este enfoque es muy prometedor para acelerar y mejorar la precisión del diseño y desarrollo de nuevas moléculas para aplicaciones farmacológicas. Aumentando la probabilidad de sacar al mercado terapias innovadoras y eficaces que, en última instancia, beneficiarán a pacientes de todo el mundo.